We use cookies to personalize content and ads, provide social media features, analyze our traffic, share information with our third-party partners or allow our third-party partners to collect information about your use of our site. For more information regarding our privacy practices and use of cookies, review our Privacy Policy.

Frontotemporal Dementia (FTD) upliFT-D (PBFT02) Clinical Trial
Information for healthcare providers
About our clinical trial

Our investigational gene therapy product candidate, PBFT02, uses an adeno-associated virus serotype 1 (AAV1) viral vector to deliver a functional GRN gene, encoding progranulin (PGRN), to a patient’s cells. The vector is delivered, with CT guidance, directly to the cerebrospinal fluid by a single injection to the cisterna magna (ICM injection).1-3

The goal of this vector and delivery approach is to provide higher-than-normal levels of PGRN to the central nervous system (CNS) to overcome the PGRN deficiency seen in GRN mutation carriers with FTD. upliFT-D will assess the safety, tolerability, and efficacy of this treatment in patients with FTD caused by a GRN mutation (FTD-GRN).1-4

Download the
FTD-GRN patient brochure ![]()


Inclusion criteria1


Study description
This is a global, interventional, multicenter, open-label, single-arm, dose-escalation study of PBFT02 delivered as a one-time dose administered into the cisterna magna to patients with FTD-GRN.1
- Two dose levels of PBFT02 will be studied in patients with FTD-GRN. The study will sequentially enroll 2 cohorts1
- An optional third dose level cohort may be enrolled based on the results of the first 2 cohorts1
- This is a 5-year study, with a 2-year main study, followed by a 3-year safety extension1
Study design1,3


Genetic testing available at no cost
Genetic testing programs are available to help you confirm FTD-GRN:

Visit InformedDNA to refer your US-based patients to no-cost genetic testing and counseling†‡ ![]()

No-cost genetic testing is available in all markets with Prevention Genetics‡ ![]()
†InformedDNA no-cost genetic testing and counseling is only available to US residents.
‡While the FTD sponsored testing programs are sponsored by Passage Bio, no personal identifying information of individuals participating in these genetic testing programs will be shared with the company.


Is your patient right for the upliFT-D trial?
Have you confirmed your patient has FTD-GRN via genetic testing?
Refer patient to a sponsored
genetic testing program.Did the results confirm FTD-GRN?
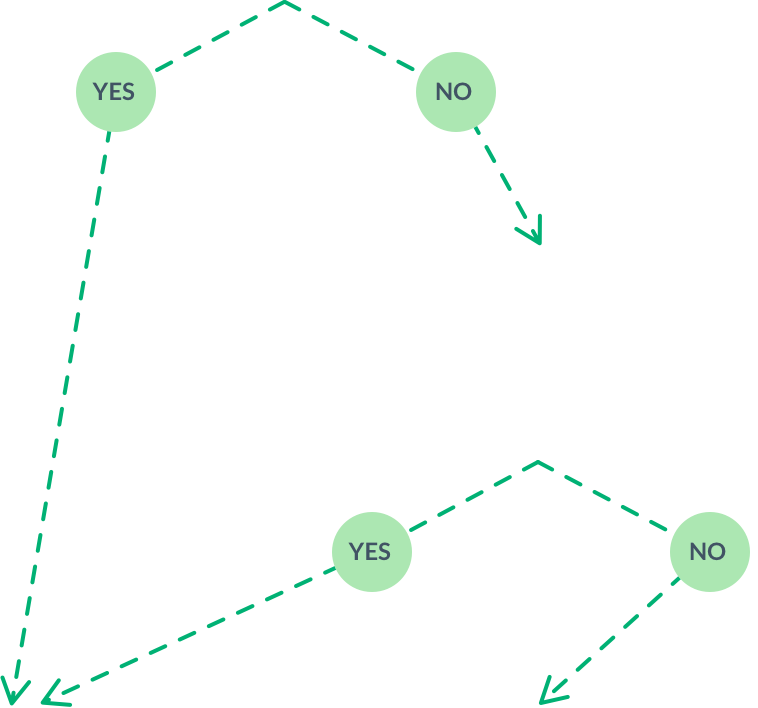
Refer patient to a trial
site location for further eligibility assessment
Patient is ineligible for
the upliFT-D trial


How to refer a patient to the upliFT-D trial
- Referring a patient to the nearest trial site can be coordinated by contacting the trial team directly to assess qualified patients for further eligibility
- Please note that all trial-related costs, including travel, will be covered by Passage Bio
References: 1. A study of PBFT02 in patients with frontotemporal dementia and progranulin mutations (FTD-GRN) (upliFT-D). ClinicalTrials.gov identifier: NCT04747431. Updated June 23, 2023. Accessed August 28, 2023. https://clinicaltrials.gov/study/NCT04747431 2. Perez BA, Shutterly A, Chan YK, Byrne BJ, Corti M. Management of neuroinflammatory responses to AAV-mediated gene therapies for neurodegenerative diseases. Brain Sci. 2020;10(2):119. doi:10.3390/brainsci10020119 3. Data on file. Passage Bio; Philadelphia, PA. 4. Anderson KM, McNeil E, Weinstein DA, Romano G. Gene therapy for treatment of monogenic neurodegenerative diseases. Neurol Reviews. 2021;(suppl):S1-S7.









