We use cookies to personalize content, provide social media features, analyze our traffic, share information with our third-party partners or allow our third-party partners to collect information about your use of our site. For more information regarding our privacy practices and use of cookies, review our Privacy Policy.
upliFT-D trial key
- Name: upliFT-D
- Treatment: PBFT02
- Goal: Study the effectiveness and safety of a new gene therapy, PBFT02, in people with FTD caused by a GRN or C9orf72 mutation
- Ages eligible: 35 to 75 years old
Common questions about this trial, participation, and frontotemporal dementia (FTD)
Clinical trials
Clinical trials are research studies where doctors see if a potential treatment is safe and effective in people.
All of the prescription therapies available to you today have been studied in clinical trials before approval.
- Clinical trials study potential new treatments
- Clinical trials are voluntary
- Participation could help others with the disease by advancing research
- People with an illness or disease also take part to help others, but also to possibly receive the newest treatment and to have added (or extra) care and attention from the clinical trial staff
- Clinical trials offer hope for many people and a chance to help researchers find better treatments for others in the future


upliFT-D trial
For the clinical trial upliFT-D, there are clinical trial sites around the world. Should you qualify for the trial, Passage Bio will work to assist you with travel to make sure you can participate in the trial. You do not need to live close to a clinical trial site in order to participate in the trial.
Participants and their caregivers are not able to choose which cohort they participate in. This helps scientists avoid any potential bias or imbalance by making sure each group is treated similarly outside of the differences outlined in the trial design.
There are often 3 phases of clinical trials. Phase 1 tests safety and helps determine optimal dose. Phase 2 tests effectiveness and safety. Phase 3 also tests effectiveness and safety in a larger group of patients.
In rare diseases, such as FTD-GRN and FTD-C9orf72, where there are low numbers of patients and limited treatment options, researchers often combine phases to answer more questions in a smaller group of patients. This is why upliFT-D is a Phase 1/2 trial.
The decision to participate in a clinical trial is very personal and should be based on your unique experiences and discussed with your doctor. You can contact Passage Bio for questions about the trial.


Frontotemporal dementia (FTD)
FTD is a disorder that affects the frontal and temporal lobes of the brain, areas that control personality, behavior, and language. FTD is often misdiagnosed as more common disorders, such as Alzheimer’s disease, but FTD tends to occur at a younger age (40-65 years) than Alzheimer’s.
FTD presents as a rapidly worsening clinical syndrome. Changes in personal and social conduct occur in the early stages of the disease and may include:
- Loss of inhibition
- Apathy
- Social withdrawal
- Hyperorality (mouthing of objects)
- Ritualistic compulsive behaviors
Symptoms are severely disabling and may lead to misdiagnosis as a psychological or emotionally-based problem, or, in the elderly, be mistaken for withdrawal or eccentricity.
FTD may progress to immobility and loss of speech and expression. Survival averages 8 years after the onset of neurocognitive deterioration.
There are currently no disease-modifying therapies approved for the treatment of FTD. Passage Bio is investigating PBFT02, a novel gene therapy for FTD-GRN and FTD-C9orf72.
The cause of FTD is usually unknown. However, scientists have determined that it is a strongly heritable disease. This means that genetics, or family history, are likely to determine the development of the disease. Up to 40% of all people with FTD have a family history of dementia.
Genes are segments of genetic material that contain codes. These codes instruct the cells in our bodies how to function. A change in the genetic code of a gene is called a mutation, and it can affect how cells function.
A majority of people who have FTD with a known genetic cause have mutations in 3 genes:
- GRN (also called a progranulin gene)
- MAPT
- C9orf72
These mutations are autosomal dominant. This means that if someone receives a mutated gene from at least one parent, they can get the disease. Additionally, it is likely that one parent has the disease.
The GRN gene tells cells how to produce the protein progranulin (PGRN). People who have FTD caused by a GRN mutation (FTD-GRN) have a mutation called a “loss of function” mutation. This means that the mutation stops cells from producing PGRN, resulting in neurodegeneration. Mutations in C9orf72 are similarly associated with decreased production of PGRN.
PGRN plays an important role in regulating cell growth, survival, repair, and inflammation in the central nervous system. Scientists are not exactly sure how a lack of PGRN causes neurodegeneration. However, people with FTD-GRN have 30%-50% lower PGRN protein levels than people without the mutation.
If you have FTD, it is important to receive genetic counseling and testing. Genetic testing can help you and your doctor:
- Determine if your FTD was caused by a mutation
- Understand more about your symptoms or disease course
- Identify clinical trials that you may be eligible for
This trial, upliFT-D, is designed for people with FTD caused by a GRN mutation (FTD-GRN) or a C9orf72 mutation (FTD-C9orf72). If you have been diagnosed with FTD and are interested in genetic testing, there may be resources to help.
If you don’t know your or your loved one’s mutation, there are free testing resources available. Ask your doctor about your testing options.
If you are a US resident, InformedDNA is providing no-cost genetic testing and counseling to people diagnosed with FTD.*

Visit InformedDNA to learn more about no-cost genetic testing and counseling for people with FTD and their loved ones.* InformedDNA![]()
While the InformedDNA FTD testing program is sponsored by Passage Bio, no personal identifying information of individuals participating in this genetic counseling and testing program will be shared with the company. InformedDNA no-cost genetic testing and counseling are only available to US residents. If you are not a US resident, talk to your doctor about genetic testing and counseling.


Gene therapy
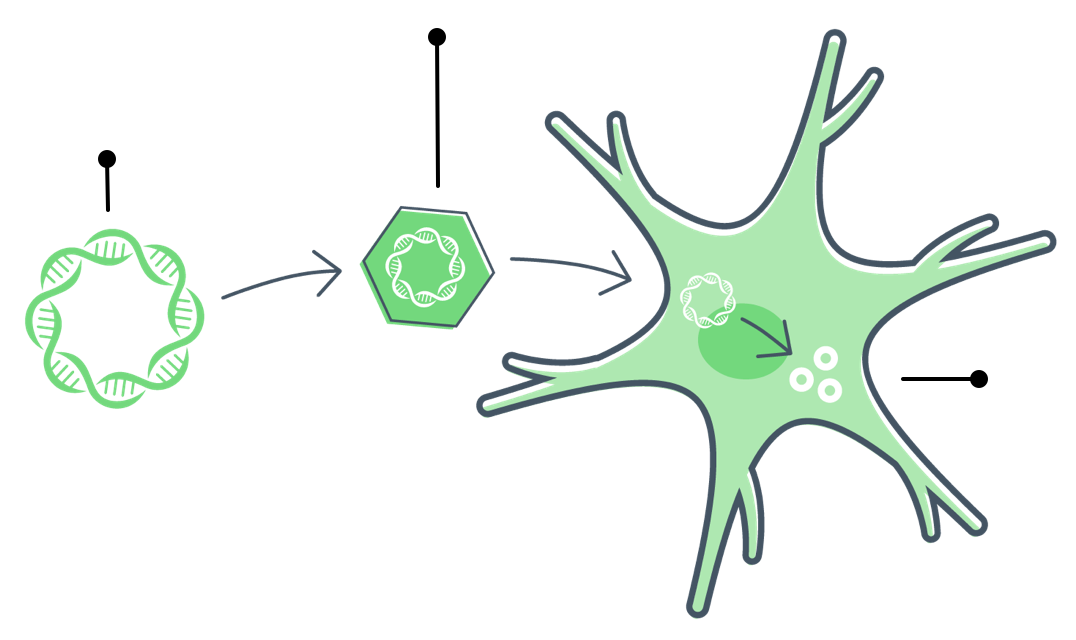
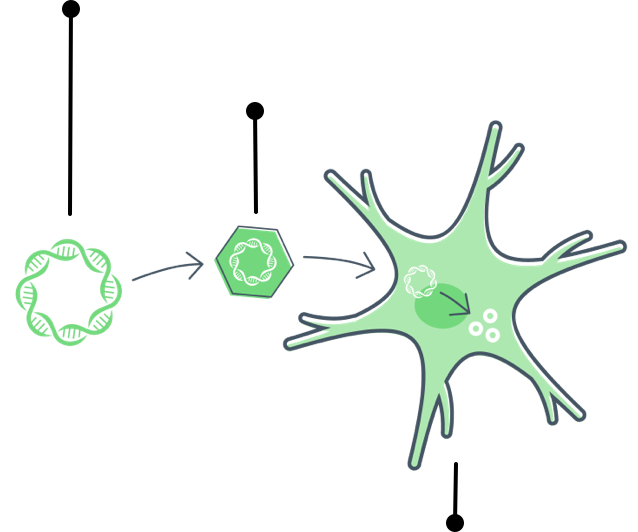
Gene therapy is a type of treatment where doctors introduce a new, working gene to the body to replace a gene that isn’t working. The body reads the new gene and produces a protein to help correct a disease.
Gene therapy starts with a healthy gene that can replace a gene that isn't working (called a mutated gene).
Gene therapy for FTD introduces a healthy gene that can instruct the body to produce progranulin.
Doctors then introduce that gene to the body.
Cells read the new gene and make progranulin.